Introduction
1
Regulatory affairs isn’t getting simpler.
New guidelines, updated standards, shifting interpretations — the volume of regulatory content grows week by week, across multiple jurisdictions and authorities.
From what we’ve seen, four challenges come up again and again:
Finding the latest, authoritative documents
Verifying the accuracy of interpretations
Understanding where specific requirements come from
Protecting sensitive internal data while doing all the above
What is GxPilot?
GxPilot is a domain-specific assistant for pharmaceutical regulatory affairs. It works exclusively with official guidance documents and databases. Every answer includes a direct citation and is traceable to a primary document.
That level of rigor isn’t optional in regulatory work — it’s essential. A missed nuance or outdated reference can delay approval, or worse, lead to rejection.
Four principles guide everything we’ve built:
Accuracy — always based on the most current validated sources
Traceability — every statement is linked to a regulation
Control — users define what jurisdictions and sources are used
Privacy — your data is yours, and stays that way
Core Features
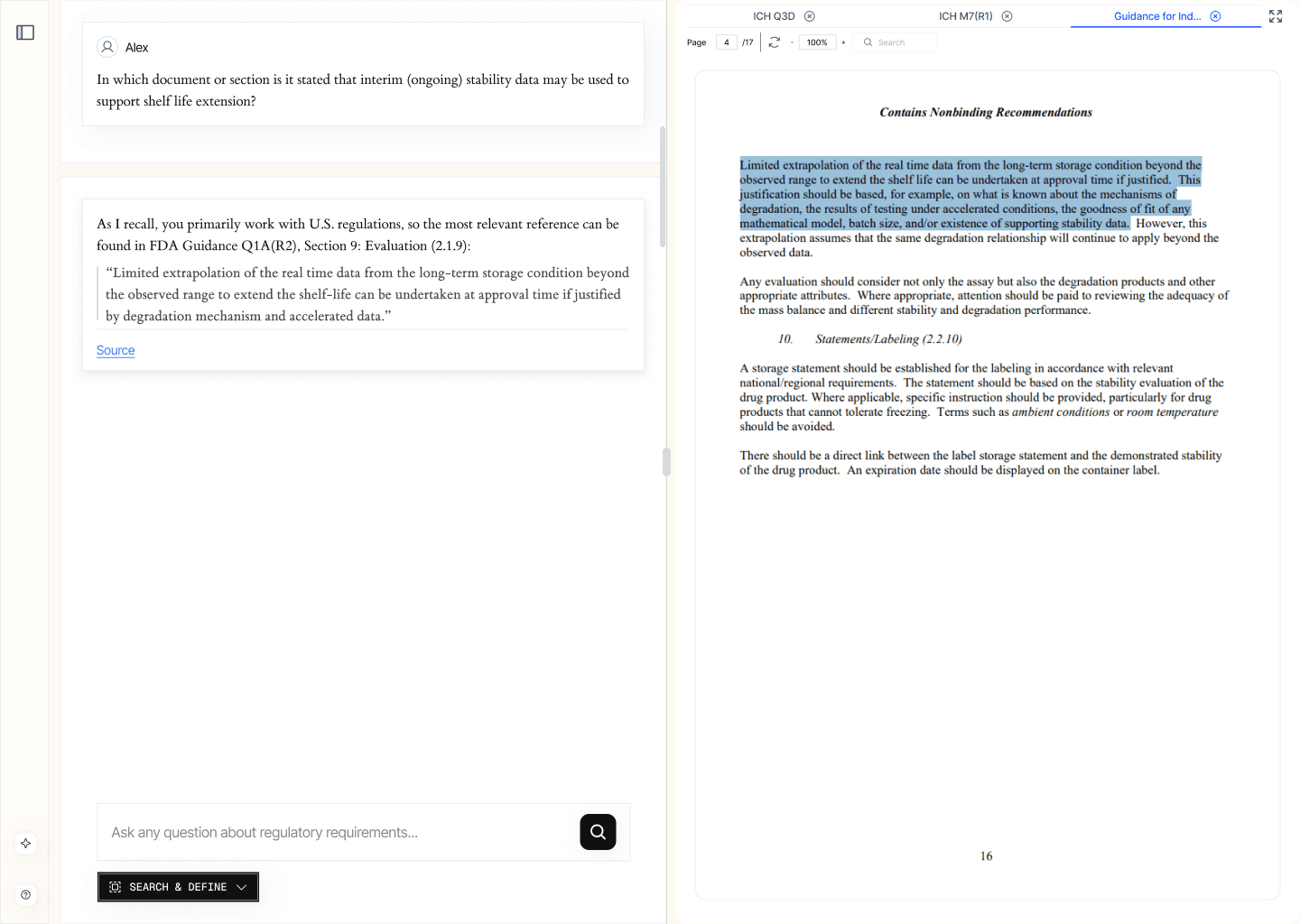
2.1 Regulatory Search (/search & define)
This function enables precise and efficient retrieval of official regulatory documentation and specific provisions. Upon entering a query — e.g., related to a registration dossier section — the system returns relevant excerpts from up-to-date regulatory sources, accompanied by direct citations.
EXAMPLE
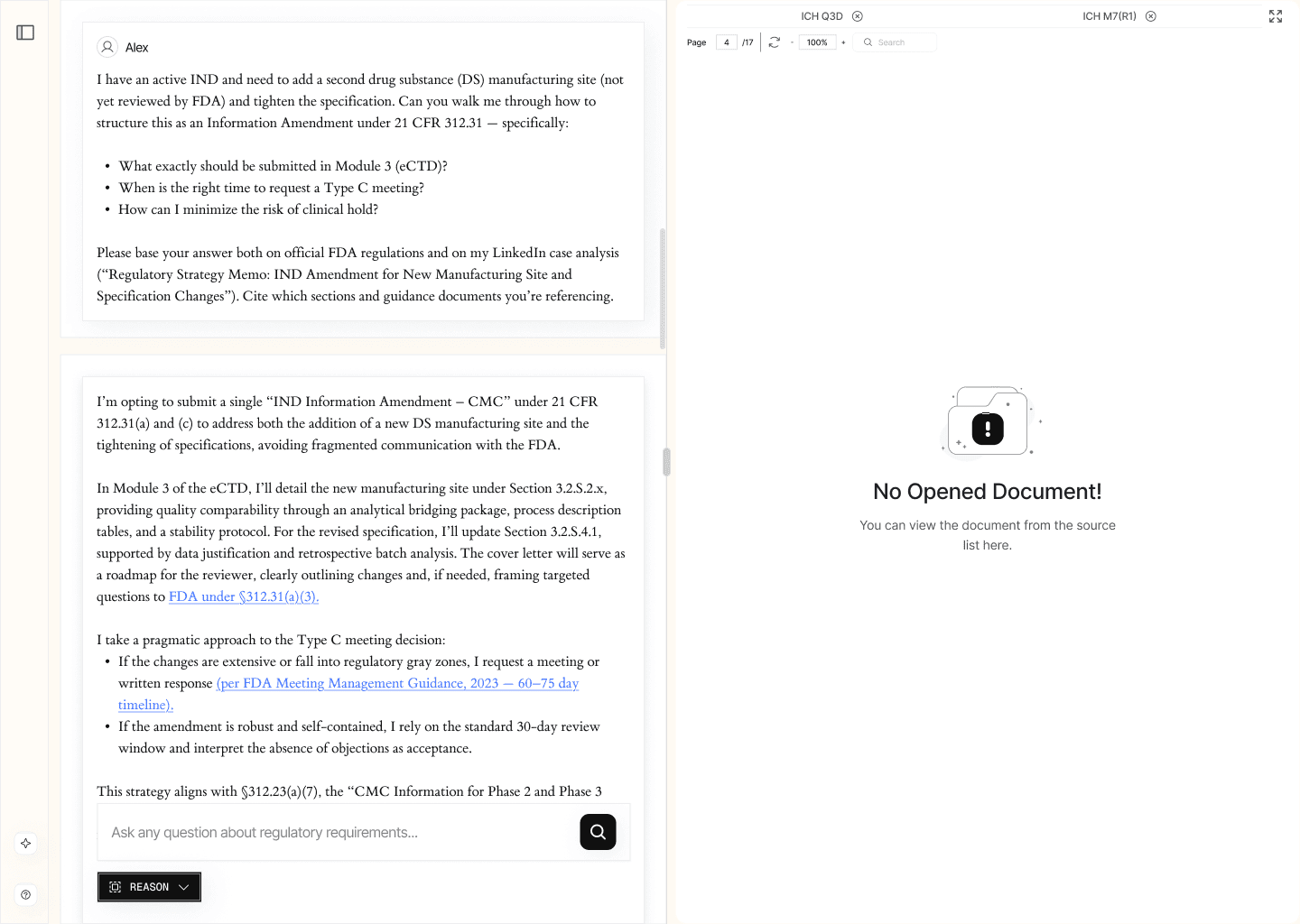
2.2. Complex Case Analysis (/reason)
Designed for use cases where regulatory decision-making requires in-depth contextual understanding rather than isolated fact-checking.
The reasoning engine analyzes the regulatory context from multiple angles, consolidating information from applicable legal frameworks, guidelines, and agency commentaries. It produces a structured explanation supported by citations from primary documents, including official clarifications from competent authorities.
EXAMPLE
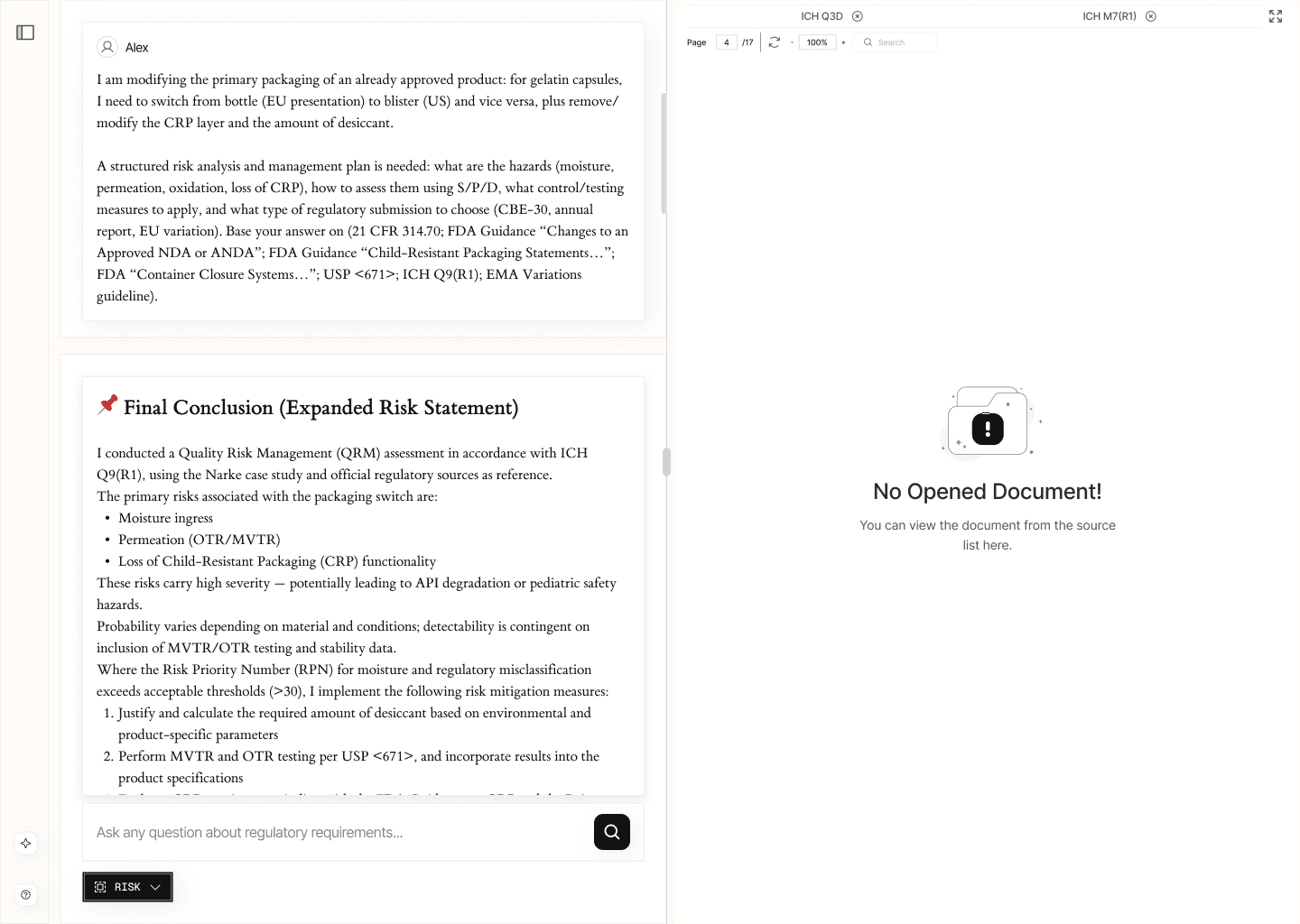
2.3. Regulatory Risk Management (/risk)
This module supports regulatory risk analysis in alignment with ICH Q9 principles.
It allows users to evaluate the regulatory risk profile associated with a specific decision — for example, changes to a dossier — based on authoritative requirements and available precedents. The system identifies potential areas of concern, explains the rationale behind each identified risk, and provides supporting excerpts from official documentation.
EXAMPLE
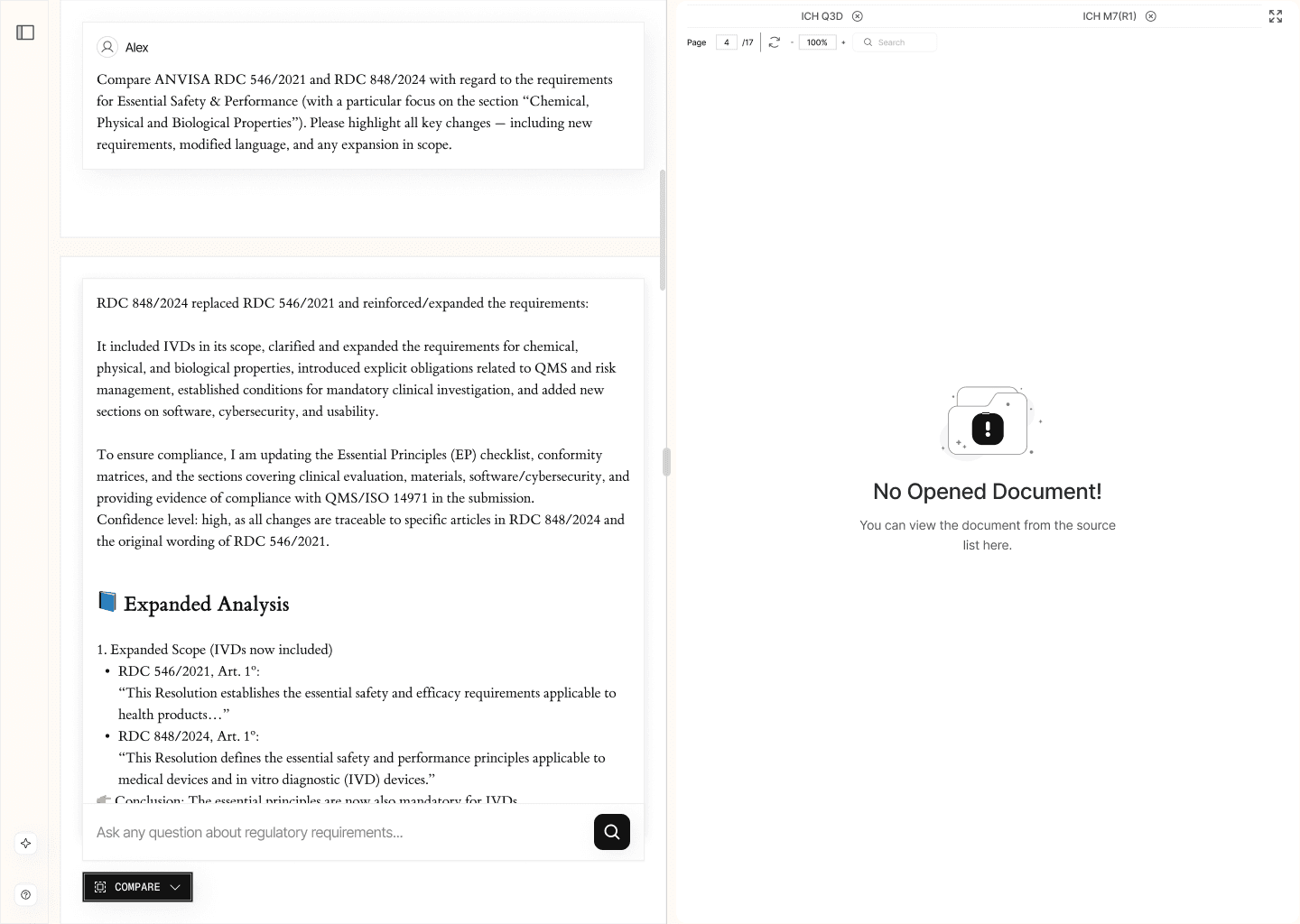
2.4. Regulatory Requirement Comparison (/compare)
This tool provides side-by-side comparisons of regulatory requirements across jurisdictions or between different versions of the same document.
It highlights both critical differences and consistent elements, ensuring that the user has a clear understanding of regulatory divergences and common standards. All comparisons are accompanied by precise source citations.
EXAMPLE
Data Sources and Security
3
GxPilot operates exclusively on official and publicly available regulatory sources, including:
FDA – United States Food and Drug Administration
EMA – European Medicines Agency
MHRA – Medicines and Healthcare products Regulatory Agency (UK)
NMPA – National Medical Products Administration (China)
ICH – International Council for Harmonisation of Technical Requirements for Pharmaceuticals
No proprietary or non-validated content is used in any part of the system’s reasoning or response generation. All outputs are source-bound and fully traceable to their originating documents.
GxPilot is also designed with enterprise-grade confidentiality by default. All user queries, prompts, and case scenarios remain fully encrypted and isolated. No query history is stored, shared, or accessible — not even by other users within the same organization.
This architecture ensures full regulatory compliance in corporate environments, including those operating under GxP, GDPR, and internal data protection policies.
How Does GxPilot Help in Daily Work?
4
From a regulatory perspective, GxPilot provides measurable benefits across core areas of day-to-day work:
Reduced time spent on document retrieval and interpretation: GxPilot enables rapid navigation through extensive regulatory corpora. This significantly accelerates operational workflows, freeing up time for high-value analytical and strategic tasks.
Improved data accuracy and source traceability: Each output is supported by explicit source citations, enabling immediate verification. This minimizes the risk of misinterpretation or reliance on outdated guidance — a critical factor in preventing errors that may delay approvals.
Streamlined regulatory risk management: The integrated risk assessment function facilitates timely evaluation of compliance risks related to dossier changes or development decisions. This allows for better-informed planning and mitigation strategies, aligned with ICH Q9 principles.
Minimization of repetitive manual tasks: By automating definition retrieval, requirement comparison, and source alignment, GxPilot reduces the cognitive load associated with routine operations — allowing regulatory experts to focus on judgment-based decision-making.
Call for Collaboration!
GxPilot is currently in active development. To make the tool as practical and helpful as possible, we need feedback from real-world regulatory specialists.
We invite you to join our alpha testing program and share your insights and suggestions. Your expertise, testing, and feedback will help us refine GxPilot into a truly valuable and effective tool for regulatory professionals.
As a thank-you for your contribution, alpha testers will receive lifetime discounted access to GxPilot upon official launch.